Atomic Number Refers To The Number Of In An Atom.
ghettoyouths
Nov 12, 2025 · 10 min read
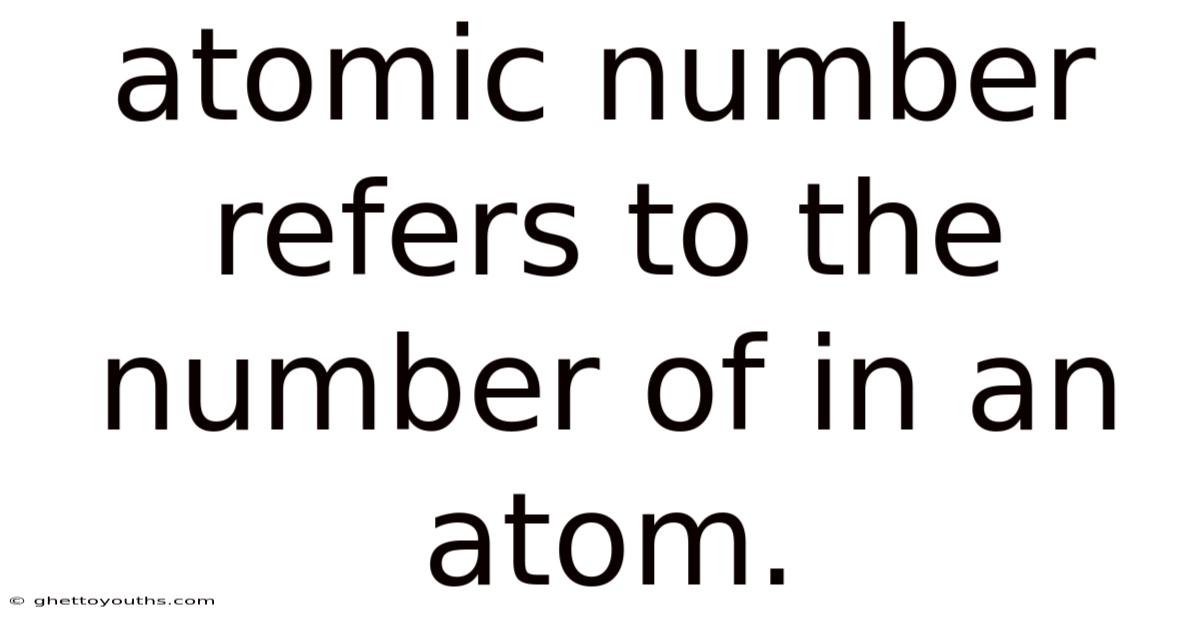
Table of Contents
Let's delve into the fascinating world of atoms and explore the significance of the atomic number, a fundamental property that defines the identity of an element. This seemingly simple number holds the key to understanding the structure, behavior, and interactions of matter at its most basic level. Understanding the atomic number is crucial for grasping the principles of chemistry, physics, and materials science.
Have you ever wondered what makes gold different from silver, or why carbon forms the backbone of all organic molecules? The answer lies within the atom, and more specifically, within its atomic number. It's a unique identifier, a fingerprint, that distinguishes one element from another.
Introduction
The atomic number is not just a random label assigned to each element; it's a direct reflection of the atom's internal composition. This article aims to provide a comprehensive understanding of the atomic number, its definition, its relationship to other atomic properties, its historical context, and its importance in various scientific disciplines. We will explore the meaning of "atomic number refers to the number of protons in an atom," and how this simple definition unlocks a vast realm of knowledge about the universe around us.
Understanding the Definition: "Atomic Number Refers to the Number of Protons in an Atom"
The atomic number, often represented by the symbol 'Z', is defined as the number of protons found in the nucleus of an atom. This definition is incredibly important because it provides a unique and unambiguous way to identify each element. Every element on the periodic table has a unique atomic number, and conversely, any atom with a specific number of protons is, by definition, that particular element.
For example, all atoms with one proton are hydrogen (Z=1), all atoms with six protons are carbon (Z=6), and all atoms with 79 protons are gold (Z=79). It's a one-to-one mapping, making the atomic number the definitive identifier of an element.
But why protons? Why not neutrons or electrons? The answer lies in the fact that the number of protons is constant for a given element. While atoms of the same element can have different numbers of neutrons (these are called isotopes), the number of protons never changes without changing the element itself. Electrons, on the other hand, can be gained or lost, resulting in ions (atoms with a net electrical charge). Therefore, the number of protons is the only fundamental property that remains constant and uniquely identifies an element.
A Comprehensive Overview: The Atom and Its Components
To truly understand the significance of the atomic number, it's essential to have a basic understanding of atomic structure. An atom consists of three primary subatomic particles:
- Protons: Positively charged particles located in the nucleus. As mentioned earlier, the number of protons defines the element.
- Neutrons: Neutral (no charge) particles also located in the nucleus. Neutrons contribute to the mass of the atom and influence its nuclear stability.
- Electrons: Negatively charged particles that orbit the nucleus in specific energy levels or shells. Electrons are responsible for chemical bonding and the interactions between atoms.
The nucleus, containing the protons and neutrons, is the dense, central core of the atom. The electrons, much lighter than protons and neutrons, occupy a much larger volume around the nucleus.
The number of electrons in a neutral atom is equal to the number of protons. This balance of positive and negative charges results in a neutral overall charge for the atom. However, atoms can gain or lose electrons to form ions, which are electrically charged.
The mass number (A) of an atom is the total number of protons and neutrons in its nucleus. Since protons and neutrons have roughly the same mass, the mass number provides an approximate measure of the atom's mass. Isotopes of an element have the same atomic number (same number of protons) but different mass numbers (different numbers of neutrons).
The Periodic Table: Organizing the Elements by Atomic Number
The periodic table is a cornerstone of chemistry, and its organization is directly based on the atomic number. The elements are arranged in order of increasing atomic number, starting with hydrogen (Z=1) and progressing to the heaviest known elements.
The periodic table's structure reflects the periodic trends in the chemical and physical properties of the elements. Elements in the same vertical column (group) have similar valence electron configurations and therefore exhibit similar chemical behavior. Elements in the same horizontal row (period) have electrons filling the same electron shells.
The atomic number is prominently displayed for each element on the periodic table, making it a quick and easy way to identify and distinguish between different elements. The periodic table is an invaluable tool for chemists, physicists, and materials scientists, providing a framework for understanding the relationships between elements and their properties.
Historical Context: The Discovery and Significance of Atomic Number
The concept of the atom has been around for centuries, dating back to the ancient Greek philosophers Leucippus and Democritus. However, the modern understanding of atomic structure and the significance of the atomic number emerged in the late 19th and early 20th centuries.
-
John Dalton's Atomic Theory (early 1800s): Dalton proposed that all matter is composed of indivisible particles called atoms, and that atoms of the same element are identical. While Dalton's theory was a major step forward, it didn't address the internal structure of the atom.
-
J.J. Thomson's Discovery of the Electron (1897): Thomson's experiments with cathode rays led to the discovery of the electron, the first subatomic particle to be identified. This discovery showed that atoms were not indivisible, but rather contained smaller, negatively charged particles.
-
Ernest Rutherford's Gold Foil Experiment (1911): Rutherford's experiment involved firing alpha particles at a thin gold foil. The results showed that most of the alpha particles passed straight through the foil, but some were deflected at large angles. This led Rutherford to propose that the atom has a small, dense, positively charged nucleus at its center.
-
Henry Moseley's Work on X-ray Spectra (1913): Moseley's experiments involved bombarding different elements with electrons and measuring the wavelengths of the emitted X-rays. He discovered a systematic relationship between the wavelength of the X-rays and the element's position on the periodic table. Moseley realized that the X-ray wavelength was related to the number of protons in the nucleus, and he proposed the concept of the atomic number as a fundamental property of each element. Moseley's work provided strong experimental evidence for the organization of the periodic table based on atomic number, and it solidified the understanding that the number of protons defines the element. Tragically, Moseley's career was cut short when he was killed in action during World War I at the young age of 27.
Moseley's contribution was revolutionary because it shifted the basis of the periodic table from atomic weight (which can be ambiguous due to isotopes) to atomic number, a fundamental and unambiguous property.
Applications of Atomic Number in Science and Technology
The atomic number is not just a theoretical concept; it has numerous practical applications in various fields:
- Chemistry: The atomic number is essential for understanding chemical bonding, chemical reactions, and the properties of chemical compounds. Knowing the atomic number allows chemists to predict how an element will interact with other elements and form chemical bonds.
- Physics: The atomic number plays a crucial role in nuclear physics, particularly in understanding nuclear reactions, radioactive decay, and the structure of atomic nuclei.
- Materials Science: The atomic number is used in materials science to design and develop new materials with specific properties. By understanding the atomic composition of a material, scientists can tailor its properties for specific applications.
- Medicine: Radioactive isotopes, which are identified by their atomic number and mass number, are used in medical imaging and cancer therapy.
- Geology: The atomic number is used in geology to determine the age of rocks and minerals using radiometric dating techniques.
Trends & Recent Developments
The study of elements and their atomic numbers is an ongoing field of research. Scientists are constantly pushing the boundaries of the periodic table by synthesizing new elements with increasingly high atomic numbers. These superheavy elements are often unstable and decay rapidly, but their synthesis and study provide valuable insights into nuclear structure and the limits of the periodic table.
Recent advancements in nuclear physics have allowed scientists to probe the structure of atomic nuclei with unprecedented precision. These studies are helping to refine our understanding of the forces that hold the nucleus together and the properties of exotic nuclei.
The development of new technologies, such as advanced mass spectrometry, has also enabled scientists to analyze the isotopic composition of materials with greater accuracy. This has important implications for fields such as forensics, environmental science, and archaeology.
Tips & Expert Advice
- Memorize the First Few Elements: Knowing the atomic numbers of the first few elements (hydrogen to neon) is extremely helpful for understanding basic chemistry concepts.
- Use the Periodic Table as a Reference: Keep a periodic table handy when studying chemistry or related subjects. It's a valuable tool for looking up atomic numbers, atomic weights, and other important information about the elements.
- Understand Isotopes: Remember that isotopes of an element have the same atomic number but different mass numbers. This distinction is important for understanding nuclear chemistry and radiometric dating.
- Relate Atomic Number to Electron Configuration: The atomic number determines the number of electrons in a neutral atom, which in turn determines its electron configuration. Understanding electron configuration is crucial for understanding chemical bonding and reactivity.
- Practice, Practice, Practice: The best way to master the concept of atomic number is to practice using it in various problems and exercises.
FAQ (Frequently Asked Questions)
-
Q: What is the difference between atomic number and mass number?
- A: The atomic number is the number of protons in an atom's nucleus, while the mass number is the total number of protons and neutrons in the nucleus.
-
Q: Can the atomic number change?
- A: No, the atomic number cannot change without changing the element itself. Nuclear reactions can change the number of protons in a nucleus, but this results in the formation of a different element.
-
Q: Why is the atomic number important?
- A: The atomic number is important because it uniquely identifies each element and determines its chemical properties.
-
Q: What is the symbol for atomic number?
- A: The symbol for atomic number is Z.
-
Q: Where can I find the atomic number of an element?
- A: You can find the atomic number of an element on the periodic table.
Conclusion
In conclusion, the atomic number, representing the number of protons in an atom, is a fundamental concept in chemistry and physics. It serves as the unique identifier for each element, dictating its position on the periodic table and influencing its chemical and physical properties. From its historical discovery to its diverse applications in science and technology, the atomic number remains a cornerstone of our understanding of the material world. Understanding "atomic number refers to the number of protons in an atom" opens the door to understanding how matter is built and how the universe works.
How has your understanding of the atomic number changed after reading this article? Are you inspired to explore more about the elements and their properties?
Latest Posts
Latest Posts
-
What Are The Knights Of Labor
Nov 12, 2025
-
How To Solve For Final Velocity
Nov 12, 2025
-
Logarithmic Functions In The Real World
Nov 12, 2025
-
Phillips Curve In Short Run And Long Run
Nov 12, 2025
-
Famous Opera House In Milan Italy
Nov 12, 2025
Related Post
Thank you for visiting our website which covers about Atomic Number Refers To The Number Of In An Atom. . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.