Which Part Of A Phospholipid Is Hydrophobic
ghettoyouths
Nov 14, 2025 · 9 min read
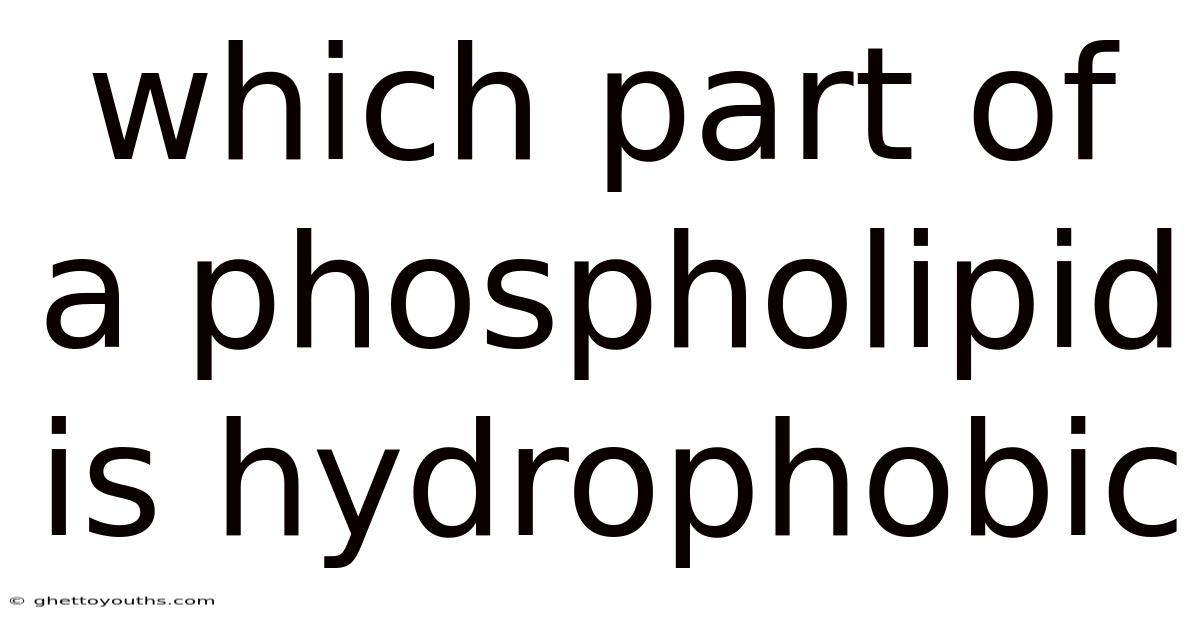
Table of Contents
Okay, here's a comprehensive article exploring the hydrophobic portion of a phospholipid, designed to be informative, engaging, and SEO-friendly:
Unraveling the Hydrophobic Nature of Phospholipids: A Deep Dive
Phospholipids are the unsung heroes of cellular life. These remarkable molecules form the very foundation of our cell membranes, acting as gatekeepers that control what enters and exits each cell. Their unique structure, a blend of hydrophilic (water-loving) and hydrophobic (water-fearing) regions, is what grants them this critical role. The key to understanding their function lies in identifying which part of a phospholipid is hydrophobic, and exploring why this characteristic is so crucial.
Understanding the hydrophobic nature of phospholipids is not just a matter of scientific curiosity; it's fundamental to comprehending how cells function, how drugs interact with the body, and how various biological processes unfold. This article will delve into the molecular structure of phospholipids, highlight the hydrophobic components, and explain the significance of this property in biological systems.
The Phospholipid Landscape: A Molecular Overview
To fully appreciate the hydrophobic aspects of phospholipids, it's essential to first understand their overall molecular architecture. A phospholipid molecule has three primary components:
-
A Phosphate Group: This is the "head" region of the phospholipid. The phosphate group is attached to a glycerol molecule and is negatively charged, making it highly polar and hydrophilic.
-
A Glycerol Backbone: Glycerol is a three-carbon alcohol that acts as the central scaffold for the phospholipid. It links the phosphate group to the fatty acid tails.
-
Two Fatty Acid Tails: These are long hydrocarbon chains that extend from the glycerol backbone. These tails are the hydrophobic heart of the phospholipid molecule.
The Hydrophobic Core: Fatty Acid Tails Exposed
The fatty acid tails are the part of the phospholipid that is hydrophobic. These tails are composed of long chains of carbon and hydrogen atoms. The carbon-hydrogen bond is nonpolar, meaning that the electrons are shared almost equally between the carbon and hydrogen atoms. This even sharing of electrons results in a lack of charge separation, making the hydrocarbon chain nonpolar.
-
Nonpolar Nature: Water molecules are polar, meaning they have a partial positive charge on the hydrogen atoms and a partial negative charge on the oxygen atom. This polarity allows water molecules to form hydrogen bonds with each other and with other polar molecules. Nonpolar molecules, like the fatty acid tails, cannot form hydrogen bonds with water.
-
The Hydrophobic Effect: When nonpolar molecules are placed in water, they disrupt the hydrogen bonding network of water molecules. To minimize this disruption, water molecules will arrange themselves around the nonpolar molecules, forming a cage-like structure. This arrangement is energetically unfavorable because it reduces the entropy (disorder) of the system. As a result, nonpolar molecules tend to cluster together, away from water. This phenomenon is known as the hydrophobic effect.
Saturated vs. Unsaturated Fatty Acids: Impact on Membrane Fluidity
The properties of the fatty acid tails, specifically whether they are saturated or unsaturated, can significantly influence the behavior of the phospholipid molecule and the overall structure of cell membranes.
-
Saturated Fatty Acids: Saturated fatty acids have carbon chains that are fully saturated with hydrogen atoms, meaning that there are no carbon-carbon double bonds. These fatty acids are straight and can pack tightly together.
-
Unsaturated Fatty Acids: Unsaturated fatty acids have one or more carbon-carbon double bonds. These double bonds create kinks in the fatty acid chains, preventing them from packing as tightly together as saturated fatty acids.
The presence of unsaturated fatty acids in phospholipids increases the fluidity of the cell membrane. The kinks introduced by the double bonds disrupt the close packing of the fatty acid tails, making the membrane more flexible and less viscous. This fluidity is essential for many cellular processes, such as:
-
Protein movement: Membrane proteins need to be able to move laterally within the membrane to carry out their functions.
-
Cell signaling: The fluidity of the membrane allows signaling molecules to diffuse and interact with receptors.
-
Membrane fusion: The fusion of membranes during processes like exocytosis and endocytosis requires the membrane to be fluid.
The Amphipathic Nature: A Balancing Act
Phospholipids are described as amphipathic molecules because they have both hydrophilic and hydrophobic regions. This unique characteristic is what allows them to form the lipid bilayer, the fundamental structure of cell membranes.
-
Formation of the Lipid Bilayer: In an aqueous environment, phospholipids spontaneously arrange themselves into a bilayer. The hydrophilic heads face outward, interacting with the water molecules on either side of the membrane. The hydrophobic tails face inward, away from the water, creating a nonpolar core.
-
Stability and Barrier Function: The lipid bilayer is a stable structure that acts as a barrier to the passage of polar molecules and ions. This barrier function is essential for maintaining the internal environment of the cell and for regulating the transport of substances across the membrane.
Comprehensive Overview: Delving Deeper
Let's delve into a more comprehensive overview of the concepts discussed above, exploring their broader implications.
1. The Significance of the Hydrophobic Effect: The hydrophobic effect is a driving force in many biological processes, including:
-
Protein folding: The hydrophobic amino acid residues in a protein tend to cluster together in the interior of the protein, away from water. This helps to stabilize the protein structure.
-
Enzyme-substrate interactions: Hydrophobic interactions can play a role in the binding of substrates to enzymes.
-
Membrane protein integration: Hydrophobic regions of membrane proteins interact with the hydrophobic core of the lipid bilayer, anchoring the protein in the membrane.
2. The Diversity of Phospholipids: There are many different types of phospholipids, each with its own unique structure and properties. Some common types of phospholipids include:
-
Phosphatidylcholine (PC): The most abundant phospholipid in most cell membranes. It has a choline head group.
-
Phosphatidylethanolamine (PE): Also abundant in cell membranes. It has an ethanolamine head group.
-
Phosphatidylserine (PS): Found primarily in the inner leaflet of the plasma membrane. It has a serine head group and carries a net negative charge.
-
Phosphatidylinositol (PI): Involved in cell signaling. It has an inositol head group.
3. The Role of Cholesterol: Cholesterol is another important lipid component of cell membranes. It is a steroid molecule with both hydrophilic and hydrophobic regions. Cholesterol helps to regulate membrane fluidity and stability.
- Fluidity Buffer: At high temperatures, cholesterol can help to prevent the membrane from becoming too fluid by interacting with the fatty acid tails and reducing their movement. At low temperatures, cholesterol can help to prevent the membrane from becoming too rigid by disrupting the close packing of the fatty acid tails.
Tren & Perkembangan Terbaru
The study of phospholipids continues to evolve with new discoveries and technological advancements. Here are some of the recent trends and developments:
-
Lipidomics: Lipidomics is a rapidly growing field that focuses on the comprehensive analysis of lipids in biological systems. Lipidomic studies are providing new insights into the role of lipids in health and disease.
-
Membrane Domains: Research suggests that cell membranes are not uniform structures but rather are organized into specialized domains with distinct lipid and protein compositions. These domains play a role in various cellular processes, such as signal transduction and membrane trafficking.
-
Drug Delivery: Phospholipids are being used to develop novel drug delivery systems. Liposomes, which are spherical vesicles made of phospholipid bilayers, can be used to encapsulate drugs and deliver them to specific cells or tissues.
-
Artificial Cells: Scientists are using phospholipids to create artificial cells, which are simplified models of living cells. These artificial cells can be used to study basic biological processes and to develop new technologies, such as biosensors and drug delivery systems.
Tips & Expert Advice
Here are some tips and expert advice for further understanding the hydrophobic nature of phospholipids:
-
Visualize the Structure: Use molecular modeling software or online resources to visualize the 3D structure of phospholipids. This will help you to better understand the arrangement of the hydrophilic and hydrophobic regions.
-
Explore the Hydrophobic Effect: Delve deeper into the concept of the hydrophobic effect by reading scientific articles and textbooks on the topic. Understanding the thermodynamic principles behind the hydrophobic effect is crucial for comprehending the behavior of phospholipids.
-
Study Membrane Models: Investigate different models of cell membranes, such as the fluid mosaic model. This will help you to understand how phospholipids interact with other membrane components, such as proteins and cholesterol.
-
Consider the Biological Context: Always consider the biological context when studying phospholipids. The properties and functions of phospholipids can vary depending on the specific cell type, tissue, and organism.
FAQ (Frequently Asked Questions)
-
Q: What makes a molecule hydrophobic?
- A: Hydrophobic molecules are nonpolar and do not interact favorably with water. They tend to cluster together in aqueous environments to minimize contact with water molecules.
-
Q: Why are the fatty acid tails of phospholipids hydrophobic?
- A: The fatty acid tails are composed of long chains of carbon and hydrogen atoms, which are nonpolar.
-
Q: How does the hydrophobic nature of phospholipids contribute to the structure of cell membranes?
- A: The hydrophobic tails of phospholipids face inward in the lipid bilayer, away from water, creating a nonpolar core that acts as a barrier to the passage of polar molecules and ions.
-
Q: What is the difference between saturated and unsaturated fatty acids?
- A: Saturated fatty acids have carbon chains that are fully saturated with hydrogen atoms, while unsaturated fatty acids have one or more carbon-carbon double bonds.
-
Q: How does cholesterol affect membrane fluidity?
- A: Cholesterol acts as a fluidity buffer, helping to prevent the membrane from becoming too fluid at high temperatures and too rigid at low temperatures.
Conclusion
The hydrophobic nature of the fatty acid tails is the defining characteristic that allows phospholipids to self-assemble into the lipid bilayer, the very foundation of cell membranes. Understanding the interplay between the hydrophilic head and the hydrophobic tails is crucial for grasping the fundamental principles of cell biology and the many processes that depend on the unique properties of these remarkable molecules.
How do you think the study of phospholipids will impact future medical advancements? Are you intrigued to explore further into the world of cellular biology?
Latest Posts
Latest Posts
-
A Steroid Hormone Binds To An Intracellular Receptor
Nov 14, 2025
-
Is Insurance Expense An Operating Expense
Nov 14, 2025
-
What Is The Emf Of A Battery
Nov 14, 2025
-
Are Black Women The Most Educated
Nov 14, 2025
-
Acura Of The Rio Grande Valley
Nov 14, 2025
Related Post
Thank you for visiting our website which covers about Which Part Of A Phospholipid Is Hydrophobic . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.